
Vaccine manufacturing & supply
Vaccines are highly specific products that are distinct from drugs in a number of ways. They are administered to a large amount of healthy people, which has implications for the benefit-risk assessment. Vaccines are also very complex biological products with lengthy manufacturing, control and release processes. This makes the production of vaccines a time-consuming and complicated process, that usually takes from 12 to 36 months, with 70% of that time dedicated to quality control. For example, our analysis finds that the seasonal influenza vaccines take around 12 months to produce, the monovalent hepatitis B vaccine takes 12-18 months, and complex multivalent vaccines take from 18 to over 36 months. For this reason, estimating demand for vaccines early is crucial to ensure adequate supply. Any disruptions to the vaccine ecosystem, e.g. border controls affecting vaccine ingredients deliveries, can lead to delays.
The fact that millions of COVID-19 vaccine doses have been manufactured and distributed all over the world since the approval of the first vaccine in 2020 has been possible thanks to the unparalleled collaboration of EU institutions, governments, health systems, regulators, and the research-based pharmaceutical industry. The COVID-19 pandemic has put an emphasis on the importance of having a resilient supply chain that would enable routine vaccines to be continuously delivered, and would support the supply of vaccines in times of crisis.
The supply chain is complex and faces many challenges. Vaccines are one of the most complex biological products to manufacture but also an invaluable public health tool. Before the COVID-19 pandemic, several vaccine shortages happened with very variable root causes. Preventing any vaccine shortage is of paramount importance and its risk factors need to be urgently addressed. For this reason, in 2022, members of Vaccines Europe published a research paper identifying 6 root causes of vaccine supply shortages in the EU.
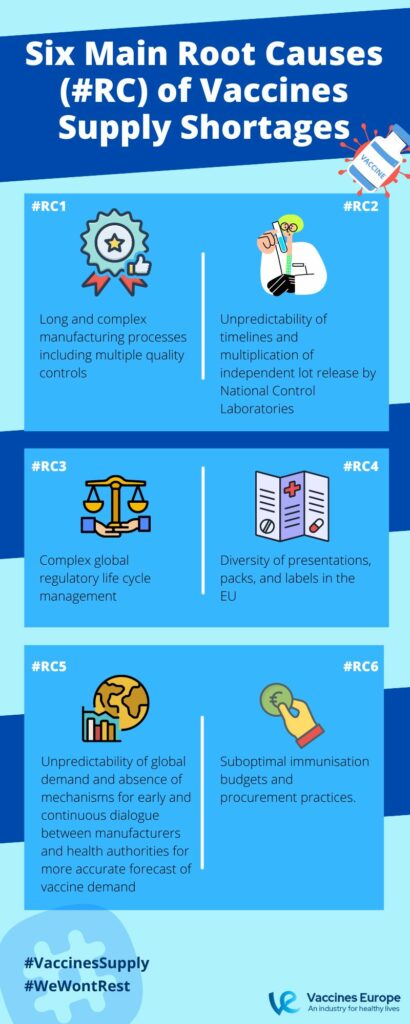
Our recommendations for addressing vaccine shortages in the EU
- Vaccine manufacturers should have good visibility of the future vaccine demand.
- Mutual recognition agreements between EU and non-EU authorities to avoid duplication of testing for well-established vaccines produced with robust manufacturing processes and strong pharmaceutical quality systems.
- Developing science- and risk-based approaches, worldwide convergence and harmonisation of regulatory requirements and procedures, as well as developing more recognition and reliance processes across regulators.
- Adopting a common EU packaging and replacing the printed Patient Information Leaflet with an electronic leaflet available online in the 24 official EU languages.
- Early and continuous dialogue between manufacturers and health authorities.
- Increased investment in vaccination budgets for inclusive and sustained immunisation plans.





