Vaccines market access
Vaccination is the foremost preventive measure to protect populations against the consequences of the spread of infectious diseases, saving millions of lives globally and generating broad public health and economical value. However, the benefits of vaccination rely on population-wide access to vaccines, targeting an entire birth cohort or large adult population, which is generally secured via government-funded national immunisation programs.
The success of a vaccination programmes is, on the other hand, tied to the willingness of policy makers to expand and finance the immunisation programme, procurement and delivery of vaccines, the monitoring of their effects in terms of vaccination coverage rates.
This is why vaccines market access pathways are different to other medicines, and yet so critical.
As Vaccines Europe, our mission is to generate new data and strong knowledge-base at the European level to articulate the full value of vaccination, the need for greater resilience of the vaccine ecosystem and its flexibility to integrate new vaccines in national immunisation programmes (NIPs).
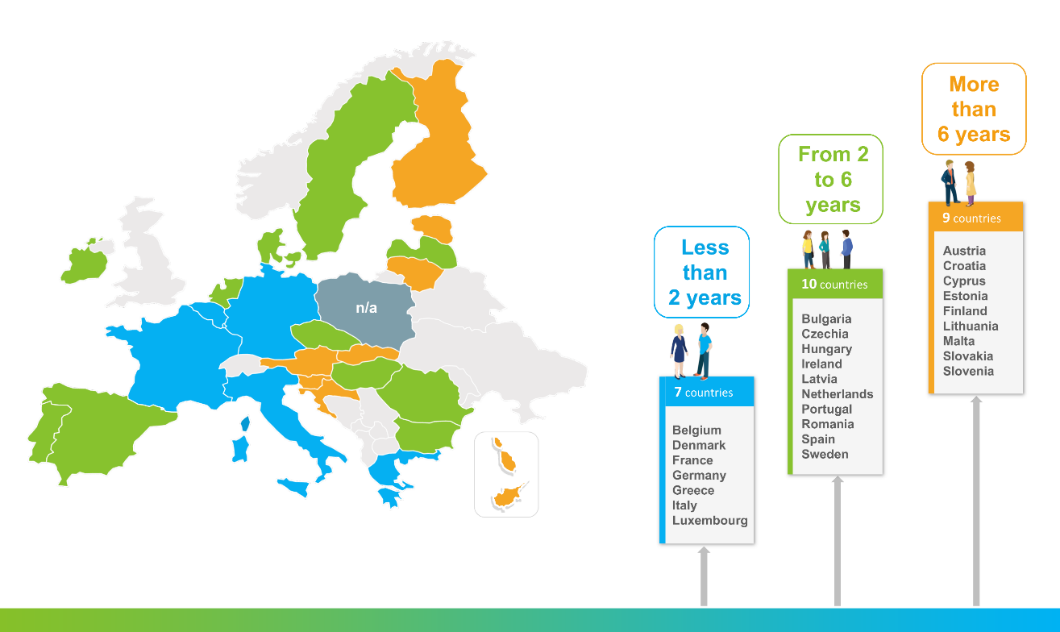
The time it takes for a vaccine to reach the population after its authorisation varies across the different EU countries – with the median time being 6 years, as identified in this research paper we published in 2021. In contrast, patient access for new innovative therapies varies between 4 months and 2.5 years in Europe1. One of the main reasons behind this significant delay for vaccines is the complexity of the market access pathways.
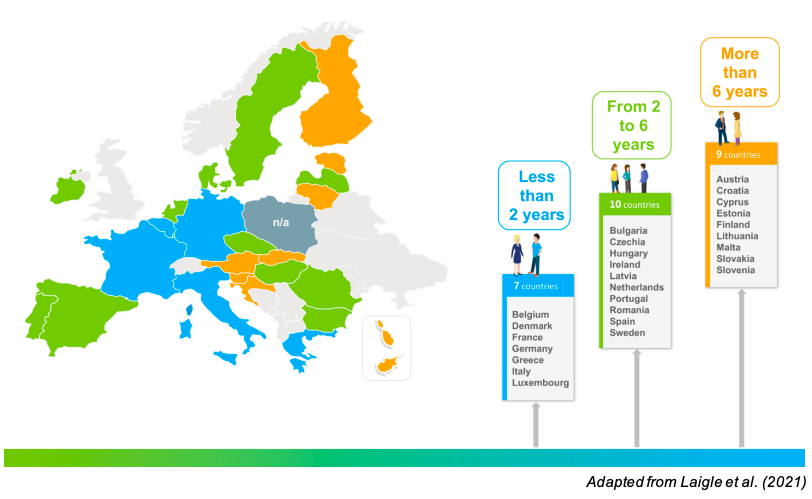
There are multiple steps that need to be performed for a vaccine to be included in the National Immunisation Programme of an EU country, and each Member State has its own specificities and differences in this process. The heterogeneity and lack of transparency of the market access pathways need to be addressed.
Strengthening the design and functioning of vaccine market access pathways can reduce the time and fight inequalities in access to vaccines for the population, support the shift towards life-course immunisation, improve vaccine confidence and support building resilient immunisation systems. We recommend embracing 4 principles to enhance vaccine assessment and decision-making pathways: timeliness, inclusiveness, consistency and transparency.

Procurement of vaccines is tender-driven in majority of EU Member States, with “one-winner takes it all” tenders usually conducted at the national level.
The value of vaccines is often taken for granted and tendering practices do not always reflect the value of the vaccination program beyond the vaccine itself, or consider the length and complexity of vaccines manufacturing.
A manufacturer who loses a public bid loses access to the market for the duration of the tender; which may last for several years. Such situation does not foster competition, neither EU supply sustainability and autonomy.